
time graphģ) A runner runs at a pace of 5 m/sec for 10 minutes, then 7 m/sec for 5 minutes, then slows to 3 m/sec for 15 minutes. time graph - Finding speed and average speed from a distance vs. Lab 9: Uniform Motion - Measuring average speed - Recognizing constant speed on a distance vs.
(This is the amount of water the barge displaces.)ĭensity = 1000 kg/m3 (from lab 8) mass = density × volume = 1000 kg/m3 × 500 m3 = 500,000 kg c) Subtract the mass of the barge from your answer in b) this is the remaining capacity of the barge in kg.ĥ00,000 kg – 20,000 kg = 480,000 kg d) Use your mass from c) and the density of steel to solve for the volume of the steel.įirst, convert the mass to grams to get similar units: mass = 480,000 kg × 1000 g/kg = 480,000,000 g volume = mass / density = 480,000,000 g / 8 g/cm3 = 60,000,000 cm3 There are 1,000,000 cm3 in a m3, so this equals 60 m3.

Volume = (20 × 5 × 5)m3 = 500 m3 b) Using the density of water in kg/m3, find the mass of water the barge could hold. If steel has a density of 8 g/cm3, what is the maximum volume of steel can the barge hold without sinking? This can be done in steps: a) Find the volume of the barge in m3. Volumecopper = volumelead masscopper / densitycopper = masslead / densitylead masscopper / masslead = densitycopper / densitylead = 9.0/11.3 = 0.796Ģ) An empty barge with a mass of 20,000 kg measures 20 m ✕ 5 m ✕ 5 m carries steel scraps. Mass = 11.3 g/cm3 × (2 cm × 2 cm × 2 cm) = 90.4 gĬopper cube: mass = 9.0 g/cm3 × (2 cm × 2 cm × 2 cm) = 72.0 g ratio = copper mass / lead copper mass = 72.0 g / 90.4 g = 0.796 (1/0.796 = 1.25 is also an okay answer, for the opposite ratio) The tricky (but short) way to do this problem: Recognize both volumes are equal and equate the volumes: Density = mass / volume The easy (but long) way to do this problem: Density = mass / volume lead cube: (Note the corrected typo above - you probably figured that out yourself though.) If you have a cube of lead 2 cm on each edge, and a cube of copper 2 cm on each edge, what would be the ratio of the masses of the 2 cubes?

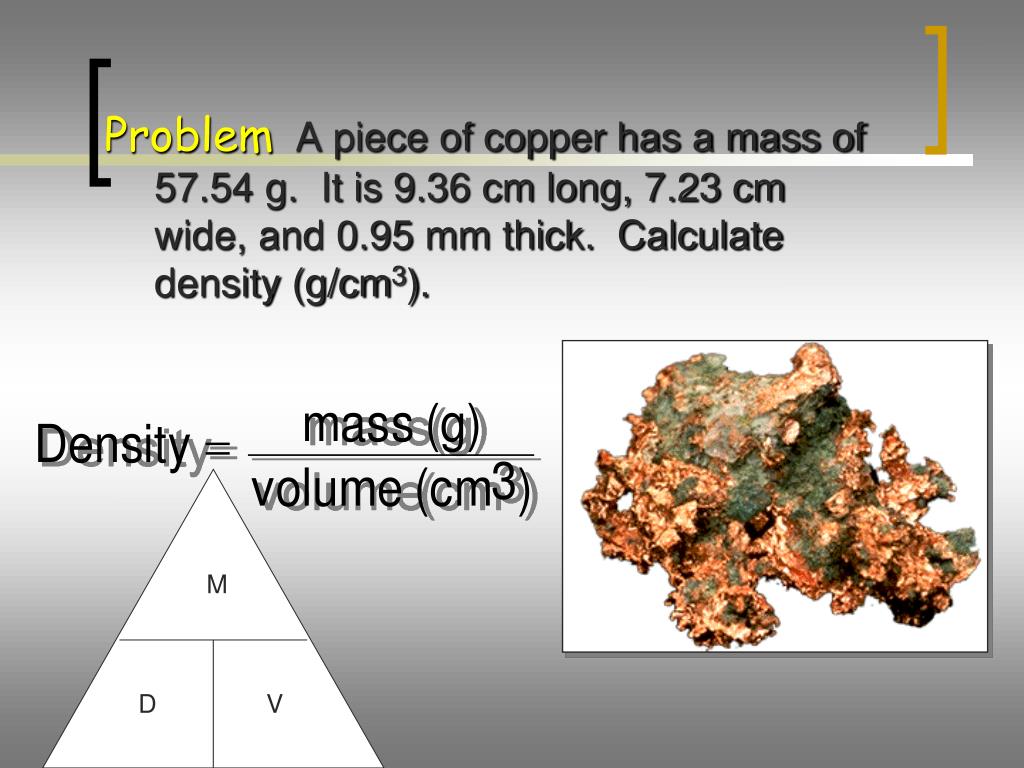
This is the calculated mass of the object which you can convert into any unit of mass measurement.Lab 8: Force and weight Density - Measuring force and weight - Determining density - Relating mass, volume and density 1) Copper has a density of about 9.0 g/cm3. The formula used by this calculator to determine mass from volume and density is:Įnter volume of the object and select the appropriate volumetric units Density of SubstanceĮnter the known density of the material being measured.
A conversion scale for volume versus mass at a fixed density will also be displayed which will relate to each calculated result. This calculator is used to determine the mass of an object from the measured volume and known density.


 0 kommentar(er)
0 kommentar(er)
